All product descriptions and articles provided on this website are intended strictly for informational and educational purposes. Our products are designed exclusively for in-vitro research (i.e., experiments conducted outside of a living organism, typically in glassware such as test tubes or petri dishes). These compounds are not approved by the FDA for use in humans or animals. They are not medications, nor are they intended to diagnose, treat, prevent, or cure any disease or medical condition. Any bodily administration-human or animal-is strictly prohibited by law. Our products are not for human consumption under any circumstances.
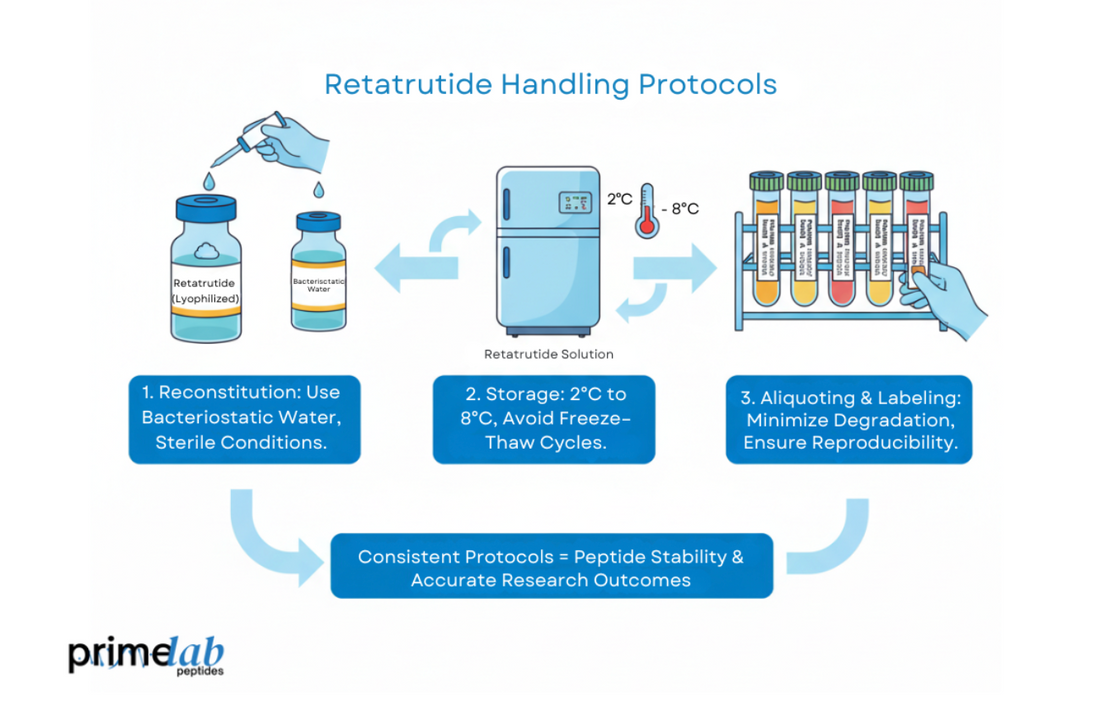
Handling Retatrutide in research requires precise and consistent protocols to ensure peptide stability and accuracy. To begin with, reconstitute lyophilized Retatrutide using bacteriostatic water under sterile conditions to prevent contamination. Afterward, store the peptide between 2°C and 8°C to preserve potency while avoiding freeze–thaw cycles. Furthermore, proper labeling and aliquoting minimize degradation, ensuring reproducibility and reliable outcomes throughout the experimental process.
At Prime Lab Peptide, we recognize the complexities researchers encounter when ensuring peptide stability and precision. Therefore, our peptides are exclusively developed for research use and tested under strict laboratory standards. As a result, we deliver exceptional consistency, traceability, and reliability to support reproducible outcomes across advanced scientific investigations.
What Makes Retatrutide a Complex Molecule to Handle in Research?
Retatrutide is complex to handle because of its multi-agonist peptide structure and sensitivity to environmental stressors. It responds rapidly to changes in temperature or humidity, demanding precise laboratory control at every stage of research. Therefore, consistency in protocols ensures accurate experimental outcomes.
Here’s why this complexity truly matters:
- Highly hygroscopic lyophilized form, sensitive to moisture and temperature.
- Multi-receptor agonist activity amplifies effects of minor structural changes.
- Low solution stability, causing faster degradation after reconstitution.
Hence, standardized documentation and strict procedural compliance are vital for maintaining stability and reproducibility. As emphasized by Princeton University’s[1] Department of Molecular Biology, peptides must remain free from non-volatile salts and be handled under tightly controlled preparative conditions.
How Should Retatrutide Be Stored to Maintain Molecular Stability?
Retatrutide must be stored under precisely controlled temperature, humidity, and light conditions to preserve its molecular stability and prevent degradation. As confirmed by the Stanford University[2] Proteomics & Nanotechnology (PAN) Facility, lyophilized peptides are recommended to be stored at −20 °C or lower for long-term preservation.
Let’s break down the essential storage principles clearly:
- Dry (lyophilized) storage: Keep at ≤ −20 °C or ideally −80 °C in tightly sealed, desiccated vials. This minimizes moisture exposure and oxidation, maintaining molecular integrity over time.
- Short-term storage: Maintain at 4 °C in a dark, low-humidity environment for temporary use. Constant humidity or temperature fluctuations can accelerate peptide instability.
- Post-reconstitution (solution form): Store between 2–8 °C and use within 2–4 weeks. Avoid repeated freeze–thaw cycles, as these can cause denaturation and unpredictable loss of activity.

How Do Handling Errors Impact Retatrutide Research Outcomes?
Handling errors can significantly impact Retatrutide research outcomes, primarily by degrading peptide integrity. For instance, repeated freeze-thaw cycles or exposure to humidity can cause fragmentation, thereby reducing the effectiveness of the active peptide. These issues lead to unreliable dose responses and inconsistent assay results, ultimately compromising the reliability and reproducibility of the data.
Moreover, errors such as improper dilution or adsorption losses can distort dose-response curves and lead to non-comparable results. Exposure to heat, light, and moisture accelerates hydrolysis and oxidation, particularly affecting sensitive residues like Met, Cys, and Trp. Contamination from microbial or enzymatic sources can further degrade peptides or interfere with bioassays. Therefore, strict adherence to protocol is vital for maintaining the integrity of research data and ensuring that findings are both reproducible and defensible.
What Quality-Control Measures Ensure Experimental Consistency?
Quality control for Retatrutide research ensures experimental consistency by confirming peptide identity and purity, documenting every handling step, and enforcing rigorous laboratory standards. According to the University of Colorado Anschutz[3] Medical Campus Proteomics Core Facility guidelines, samples must be submitted under clear documentation and prepared free of interfering contaminants.
Now explore the three essential quality-control components:
1. Analytical Validation and Purity Assurance
Each Retatrutide batch must undergo thorough testing using HPLC or mass spectrometry to confirm purity and identity. These analyses quickly identify any degradation or contamination. Consequently, consistent validation enhances accuracy, strengthens reproducibility, and ensures dependable experimental outcomes.
2. Documentation and Traceability Standards
Accurate documentation is fundamental for maintaining reproducibility and accountability. Researchers should log every process, including storage conditions and reconstitution details, to ensure complete transparency. As a result, traceability supports data integrity and enables teams to identify experimental inconsistencies easily.
3. Compliance and Laboratory Integrity
Strong compliance practices guarantee precision and safety in Retatrutide research. Regular equipment calibration ensures consistent measurements and minimizes technical errors. Furthermore, adherence to institutional and biosafety protocols maintains standardized procedures, fostering reliable and ethically sound scientific investigations.
Advance Your Research Precision with Expert Solutions from Prime Lab Peptide
Researchers often face persistent challenges such as maintaining peptide stability, minimizing degradation, and ensuring reproducibility across experiments. Slight variations in handling or storage can lead to inconsistent data and wasted resources. Moreover, the growing complexity of peptide-based studies demands precise formulation, documentation, and quality assurance to achieve dependable results.
At Prime Lab Peptide, we address these research challenges through rigorous standards and controlled synthesis of Retatrutide and other research-use-only peptides. Each batch is validated for purity, consistency, and traceability to ensure reliable outcomes in scientific studies. For customized solutions and technical support, contact us to discuss your specific research requirements.

FAQs
What Is the Primary Purpose of Retatrutide in Research?
The primary purpose of Retatrutide in research is to investigate multi-agonist peptide activity and receptor signaling mechanisms. It enables scientists to analyze molecular stability, interaction patterns, and reproducibility under controlled laboratory conditions, supporting accurate and consistent experimental outcomes.
How Should Retatrutide Be Prepared for Laboratory Use?
Retatrutide should be prepared by reconstituting the lyophilized peptide with bacteriostatic water under sterile conditions. This ensures purity and prevents contamination. Moreover, careful handling during preparation preserves molecular integrity and promotes reproducible, reliable results in controlled laboratory research.
What Storage Conditions Preserve Retatrutide Stability Best?
The best storage conditions for Retatrutide are in a dry, lyophilized form at −20°C or lower. For long-term stability, it should be kept at −80°C. Additionally, protect it from light, heat, and moisture to prevent degradation.
Why Is Documentation Important When Handling Retatrutide?
Accurate documentation ensures traceability and supports data integrity throughout peptide studies. Each handling step should be logged carefully. Consequently, maintaining such detailed records improves reproducibility, transparency, and confidence in the resulting experimental data.




