All product descriptions and articles provided on this website are intended strictly for informational and educational purposes. Our products are designed exclusively for in-vitro research (i.e., experiments conducted outside of a living organism, typically in glassware such as test tubes or petri dishes). These compounds are not approved by the FDA for use in humans or animals. They are not medications, nor are they intended to diagnose, treat, prevent, or cure any disease or medical condition. Any bodily administration-human or animal-is strictly prohibited by law. Our products are not for human consumption under any circumstances.
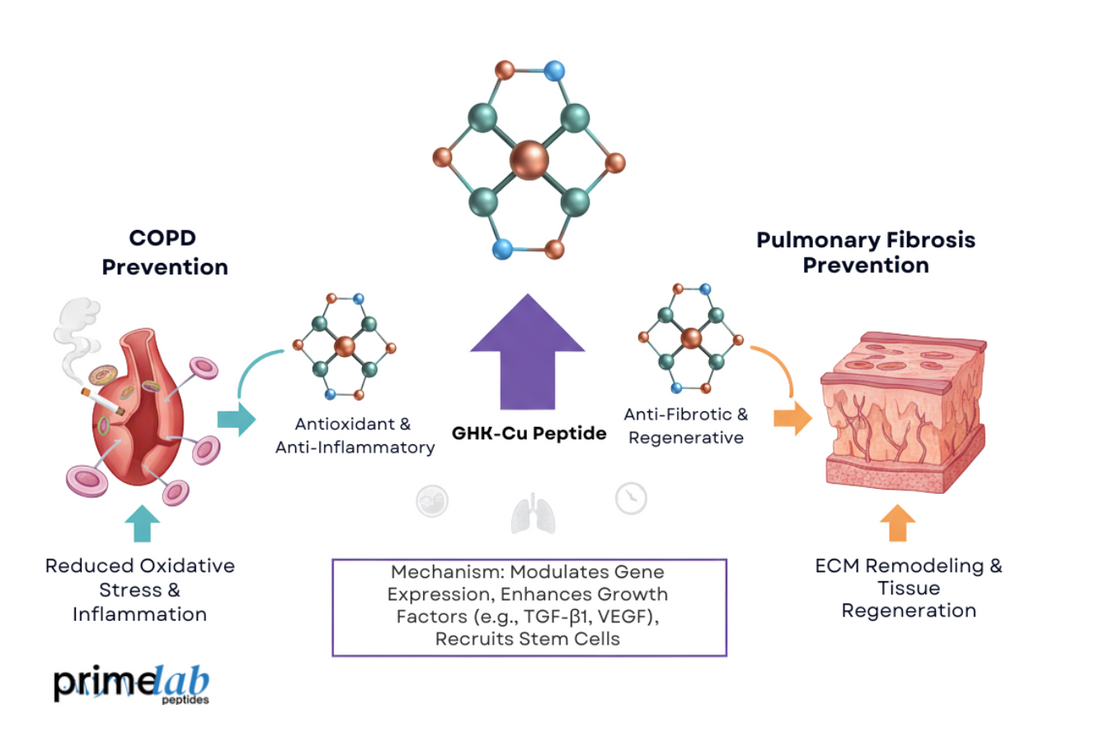
According to the Institute for Health Metrics and Evaluation[1], chronic respiratory diseases accounted for about 4 million deaths worldwide in 2019, ranking as the third leading cause of death globally. This alarming statistic highlights the urgent need for advanced peptide-based research in pulmonary repair. Within this framework, GHK-Cu has drawn significant attention for its antioxidant and anti-fibrotic potential in preclinical studies.
At Prime Lab Peptide, we support researchers by providing rigorously tested, laboratory-grade peptides designed for experimental reliability and consistency. Our focus is scientific advancement, not commercial use. Through precise formulation, verified purity, and global research collaboration, we help investigators overcome experimental variability and accelerate peptide-based discoveries in biomedical and molecular studies.
How Does GHK-Cu Influence Fibrotic Signaling Pathways?
GHK-Cu influences fibrotic signaling pathways by modulating the TGF-β1/Smad2/3 cascade, which governs collagen synthesis and epithelial-to-mesenchymal transition (EMT). Experimental studies suggest it may reduce fibroblast activation and stabilize extracellular matrix dynamics. Moreover, it appears to balance multiple molecular checkpoints, promoting structural homeostasis in lung tissue.
Mechanistic insights include:
- Inhibits Smad2/3 phosphorylation, reducing fibroblast activation effectively.
- Restored E-cadherin levels, suppressing EMT and maintaining epithelial stability.
- Balanced MMP-9/TIMP-1 ratio, supporting regulated extracellular matrix remodeling.
Furthermore, GHK-Cu has been observed to attenuate IGF-1 expression, a factor that otherwise amplifies TGF-β1 synthesis. Together, these findings suggest that GHK-Cu may act as a dual-pathway regulator, modulating both Smad-dependent and IGF-linked mechanisms in preclinical models.
What Current Research Gaps Exist in GHK-Cu Pulmonary Studies
Current research gaps in GHK-Cu pulmonary studies include limited human clinical data, inconsistent dosing protocols, and an incomplete understanding of its molecular mechanisms. The Harvard Fibrosis Network[2] at the Harvard Stem Cell Institute emphasizes the critical need for advanced mechanistic and translational research, particularly in models of pulmonary fibrosis.
To address these limitations, researchers highlight three critical focus areas:
1. GHK vs. GHK-Cu Comparison
Comparative studies distinguishing native GHK from its copper complex are scarce. Establishing differential bioactivity and stability in pulmonary models could define the specific molecular contributions of copper binding to antifibrotic and redox regulation.
2. Dose and Signaling Clarity
Data on dose-response kinetics and intracellular signaling duration remain inconsistent. Controlled in vitro and in vivo studies are necessary to determine optimal peptide concentration, exposure timing, and molecular persistence under oxidative conditions.
3. Multi-Omics and Drug Synergy
Future research should apply multi-omics profiling to map gene and protein regulation comprehensively. Additionally, exploring GHK-Cu synergy with antifibrotics, such as Pirfenidone or Nintedanib, could reveal additive effects in fibrosis mitigation pathways.

What Experimental Evidence Supports Its Role in Pulmonary Repair?
Experimental findings suggest that GHK-Cu exhibits measurable effects in preclinical models of oxidative and fibrotic lung injury. In a study conducted at China Medical University and published in Frontiers in Molecular Biosciences[3], C57BL/6 mice exposed to cigarette smoke for 12 weeks received intraperitoneal GHK-Cu (0.2–20 μg/g/day). The results showed reduced inflammatory cytokines (TNF-α, IL-1β) and partial reversal of emphysematous damage, indicating promising biological activity in lung tissue recovery.
Furthermore, histopathological evaluations revealed notable restoration of alveolar structure and a marked decrease in collagen accumulation, confirmed through Sirius Red staining. Improvements of 40–60% were documented in the chronic inflammation index compared with untreated controls. Additionally, both in vitro (A549 cell) and in vivo findings indicated reduced oxidative stress–related injury, supporting the potential reparative activity of GHK-Cu within controlled experimental conditions.
How Does GHK-Cu Regulate Inflammatory and Oxidative Stress Biomarkers?
GHK-Cu regulates inflammatory and oxidative stress biomarkers by modulating redox-sensitive signaling that maintains a balance between inflammatory and antioxidant responses. As reported in research from the University of British Columbia (UBC)[4], GHK-Cu further regulates TGF-β1 signaling and fibroblast redox homeostasis, reinforcing its experimentally observed biochemical modulation.
These molecular interactions highlight its broad biochemical modulation:
- Inhibits NF-κB activation, reducing excessive cytokine release and inflammatory signaling, which limits downstream tissue injury and prevents uncontrolled oxidative cascades in experimental lung models.
- Activates Nrf2/Keap1 signaling, enhancing transcription of antioxidant enzymes like HO-1 and SOD, thereby promoting cellular resilience and protecting epithelial cells from oxidative imbalance.
- Suppresses iNOS and MPO activity, lowering nitric oxide–driven stress and neutrophil-induced oxidation, which collectively contribute to stabilized tissue integrity under inflammatory environments.
Empowering GHK-Cu Research Innovation with Precision from Prime Lab Peptide
Researchers investigating peptides like GHK-Cu often face challenges, including inconsistent peptide quality, limited data reproducibility, and difficulty sourcing research-grade materials that meet experimental purity standards. These issues can delay progress, complicate validation across studies, and hinder accurate mechanistic exploration within the frameworks of pulmonary and molecular biology research.
At Prime Lab Peptide, we provide researchers with rigorously tested, high-purity GHK-Cu peptides specifically formulated for controlled laboratory investigations. Our dedication to analytical transparency, precision synthesis, and verified documentation ensures reproducible and credible results across experiments. For research collaborations or sourcing inquiries, we invite investigators to contact us for verified laboratory-grade peptide solutions.

FAQs
What Makes GHK-Cu a Focus in Pulmonary Research?
GHK-Cu is a focus in pulmonary research because it regulates fibrotic and oxidative pathways linked to lung injury. It modulates TGF-β1 and antioxidant responses, offering mechanistic insights. Therefore, researchers study it to understand peptide-mediated molecular regulation.
How Is GHK-Cu Typically Studied in Laboratory Models?
GHK-Cu is typically studied in laboratory models through in vitro fibroblast cultures and in vivo fibrosis models. Researchers use oxidative stress assays to observe redox modulation. These controlled systems help define their signaling impact within pulmonary tissues.
What Are the Major Challenges in GHK-Cu Research?
The significant challenges in GHK-Cu research include limited clinical translation, unstable peptide formulations, and non-standardized dosing protocols. Experimental reproducibility often varies across laboratories. Thus, uniform synthesis and characterization methods remain essential for scientific accuracy.
Why Is Peptide Purity Important in Experimental Studies?
Peptide purity is essential in experimental studies because it directly influences molecular interactions and reproducibility. Impurities can disrupt assay precision and cellular responses. Hence, maintaining verified purity ensures dependable and interpretable research findings.




































1 comment
I was diagnosed with Idiopathic Pulmonary Fibrosis (IPF) four years ago. For over two years, I relied on prescription medications and therapies, but unfortunately, the symptoms continued to worsen. My breathing became more labored, and I experienced increasing fatigue and shortness of breath with even minimal activity.Last year, out of desperation and hope, I decided to try an herbal treatment program from NaturePath Herbal Clinic.Honestly, I was skeptical at first, but within a few months of starting the treatment, I began to notice real changes. My breathing became easier, the tightness in my chest eased, and I felt more energetic and capable in my daily life. Incredibly, I also regained much of my stamina and confidence. It’s been a life-changing experience I feel more like myself again, better than I’ve felt in years.If you or a loved one is struggling with IPF, I truly recommend looking into their natural approach. You can visit their website at www.naturepathherbalclinic.com
info@naturepathherbalclinic.com