All product descriptions and articles provided on this website are intended strictly for informational and educational purposes. Our products are designed exclusively for in-vitro research (i.e., experiments conducted outside of a living organism, typically in glassware such as test tubes or petri dishes). These compounds are not approved by the FDA for use in humans or animals. They are not medications, nor are they intended to diagnose, treat, prevent, or cure any disease or medical condition. Any bodily administration-human or animal-is strictly prohibited by law. Our products are not for human consumption under any circumstances.
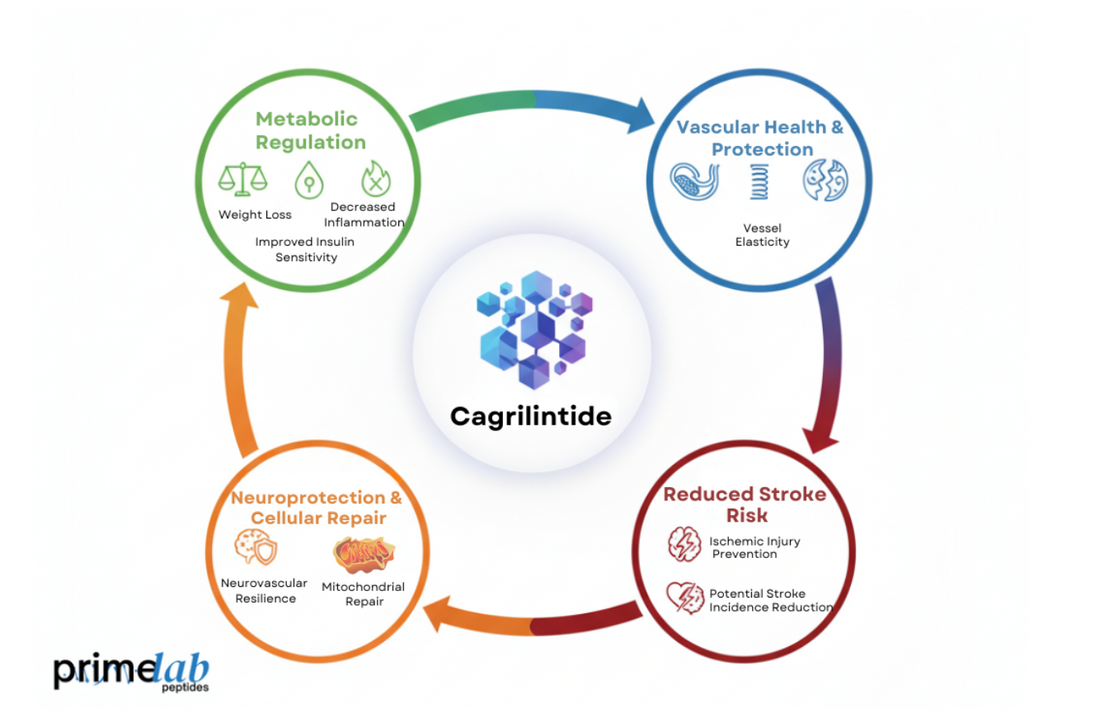
Nearly one in four strokes is linked to metabolic disorders, which makes therapies like Cagrilintide increasingly significant for research. Recent findings from the University of Alabama at Birmingham[1] show that Cagrilintide improves weight management, insulin sensitivity, and inflammation, key factors influencing stroke risk. Moreover, the university’s clinical study confirmed sustained metabolic benefits. However, despite these results, no trial has yet verified its effect on reducing actual stroke incidence.
At Prime Lab Peptide, we empower researchers to overcome challenges in metabolic and cardiovascular studies by providing high-purity, research-grade peptides like Cagrilintide. Our precision synthesis, rigorous quality testing, and reliable global supply help accelerate discovery, enabling scientists to explore innovative pathways in stroke prevention and metabolic health with complete confidence.
How Is Cagrilintide Mechanistically Linked to Stroke Prevention?
Cagrilintide is mechanistically linked to stroke prevention through its ability to improve metabolic regulation and vascular health. It activates amylin receptors that enhance glucose control, promote weight reduction, and decrease inflammation, all of which reduce strain on blood vessels and protect cerebrovascular integrity.
The following actions highlight Cagrilintide’s vascular defense pathways:
- Improves glycemic balance, minimizing endothelial stress and vascular injury.
- Promotes weight loss, which lowers blood pressure and cholesterol levels.
- Reduces inflammatory markers, supporting healthier and more flexible blood vessels.
According to the research from the University of Oxford[2] highlights how peptide-based therapies targeting inflammation support cardiovascular health. By regulating immune and vascular responses, treatments like Cagrilintide may enhance brain circulation, reduce vascular stress, and potentially lower long-term stroke risk.
What Mechanistic Pathways Explain Reduced Stroke Risk?
The mechanistic pathways through which Cagrilintide reduces stroke risk involve metabolic, vascular, and neuroprotective processes. It modulates inflammation, enhances lipid and glucose metabolism, and supports endothelial repair, collectively safeguarding brain tissue and blood vessels from ischemic injury and oxidative stress.
Together, these mechanisms explain Cagrilintide’s promising cerebrovascular impact:
1. Anti-Inflammatory and Antioxidant Action
Research from the University of Kentucky[3] shows that amylin-based therapies target vascular inflammation and oxidative stress to protect against stroke. Their project, “Amylin Vasculopathy: A Therapeutic Target to Reduce Stroke,” explores how peptide modulation preserves vascular integrity and prevents endothelial dysfunction.
2. Enhanced Lipid and Metabolic Regulation
Cagrilintide improves lipid balance and glucose control, reducing plaque buildup and atherosclerosis progression. These metabolic improvements decrease the likelihood of vessel occlusion, a major cause of ischemic stroke.
3. Neurovascular and Mitochondrial Protection
By promoting mitochondrial efficiency and cellular repair, Cagrilintide enhances neuronal survival and strengthens vascular resilience. This dual action helps reduce the severity and recurrence of stroke-related events.

How Does Current Evidence Compare Cagrilintide With Other Antidiabetic Agents?
Current evidence suggests that Cagrilintide is not yet proven but shows potential advantages over other antidiabetic agents in reducing stroke risk. While GLP-1 receptor agonists already demonstrate established cardiovascular and cerebrovascular benefits, studies on Cagrilintide are still in early phases. It has shown significant effects on weight reduction and metabolic control, which are key stroke-risk factors. However, conclusive evidence directly linking Cagrilintide to stroke prevention is still awaited.
Furthermore, a review by the Cardiovascular Research Group at Flinders University and the South Australian Health & Medical Research Institute found that GLP-1 receptor agonists significantly reduce non-fatal stroke rates and enhance vascular outcomes. Published in the Journal of Clinical Medicine[4], the study emphasized that peptide-based therapies with metabolic benefits, such as Cagrilintide, may provide comparable cerebrovascular protection, making it an important focus for future research.
What Clinical Trials Are Evaluating Cagrilintide’s Cardiovascular Outcomes?
The cardiovascular outcomes of Cagrilintide are being evaluated through large-scale global clinical trials focused on stroke prevention and overall heart health. According to the ClinicalTrials.gov[5] database, one major ongoing study is investigating its long-term effects on major adverse cardiovascular events to determine how metabolic improvements may translate into measurable vascular protection and reduced stroke risk.
These trials aim to uncover how Cagrilintide’s dual metabolic actions influence heart and brain health:
- REDEFINE 3 Trial: Focuses on reducing cardiovascular events and assessing stroke incidence through long-term metabolic intervention with Cagrilintide and semaglutide.
- Metabolic and Vascular Outcomes: Evaluates improvements in insulin sensitivity, lipid balance, and inflammatory markers to establish a link between metabolic regulation and reduced vascular risk.
- Global Collaboration: Conducted across multiple international research sites, ensuring comprehensive data on Cagrilintide’s cardiovascular safety and potential in stroke prevention.
Advancing Stroke Research with Innovative Cagrilintide Peptides by Prime Lab Peptide
Many researchers encounter barriers when advancing peptide-based stroke and metabolic research. Challenges include inconsistent peptide quality, batch variability, and limited reproducibility in preclinical data. In addition, complex regulatory requirements and supply chain limitations often slow the validation of novel compounds, delaying progress toward understanding Cagrilintide’s potential cerebrovascular and metabolic benefits.
At Prime Lab Peptide, we focus on resolving the key challenges researchers face in peptide-based vascular studies. Our Cagrilintide research peptides are synthesized with high purity and verified consistency, ensuring dependable data across experiments. Each batch undergoes rigorous analytical validation and documentation for full transparency. For collaborations or custom peptide requirements, contact us to discuss your research needs.

FAQs
What makes Cagrilintide a unique research peptide?
Cagrilintide is unique because it targets the amylin receptor to regulate metabolism and vascular health. Its dual impact on glycemic control and inflammation reduction offers valuable data for cerebrovascular studies. Therefore, it holds distinct promise for advanced metabolic research.
How does Cagrilintide support cerebrovascular studies?
Cagrilintide supports cerebrovascular research by improving endothelial function and reducing oxidative stress. These mechanisms enhance vascular resilience under metabolic strain. Consequently, it serves as a reliable model compound for exploring stroke-related biochemical pathways.
Why is Cagrilintide studied alongside GLP-1 analogs?
Cagrilintide is studied with GLP-1 analogs because their combined metabolic effects strengthen cardiovascular and neurovascular protection. The synergy improves lipid metabolism, inflammation control, and glycemic balance. Thus, this dual approach expands understanding of multi-pathway stroke prevention.
What research gaps exist in Cagrilintide’s stroke studies?
The research gaps in Cagrilintide’s stroke studies mainly concern the lack of long-term cerebrovascular trials and direct stroke outcome data. Current studies focus largely on metabolic outcomes instead of neural mechanisms. Therefore, future research must integrate advanced imaging and biomarker evaluation to confirm its neurovascular potential.
Refrences
5. International Committee of Medical Journal Editors. (n.d.). REDEFINE 3: A research study to see the effects of CagriSema in people living with diseases in the heart and blood vessels (NCT05669755). ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT05669755



































